How does volatile organic compounds concentration affect the environment

Volatile organic compounds are substances that evaporate at room temperature and are usually found in household products, cleaning products, and building materials, for example. VOC exposure in indoor environments can irritate the eyes, throat, and nose, as well as cause headaches, dizziness, and potentially lead to memory loss or visual impairment. They are also believed to contribute to ‘sick building syndrome’1.
To know what harmful chemicals to avoid, people can refer to the US EPA list of volatile organic compounds, which has been compiled for our environmental protection. The best way to keep track of your air quality indoors is with a smart indoor air quality monitor, which can notify you of harmful concentrations of VOCs. However, VOCs do not just affect our indoor environment. They also can contribute to a number of adverse environmental problems, especially in urban areas.
Environmental effects of VOCs
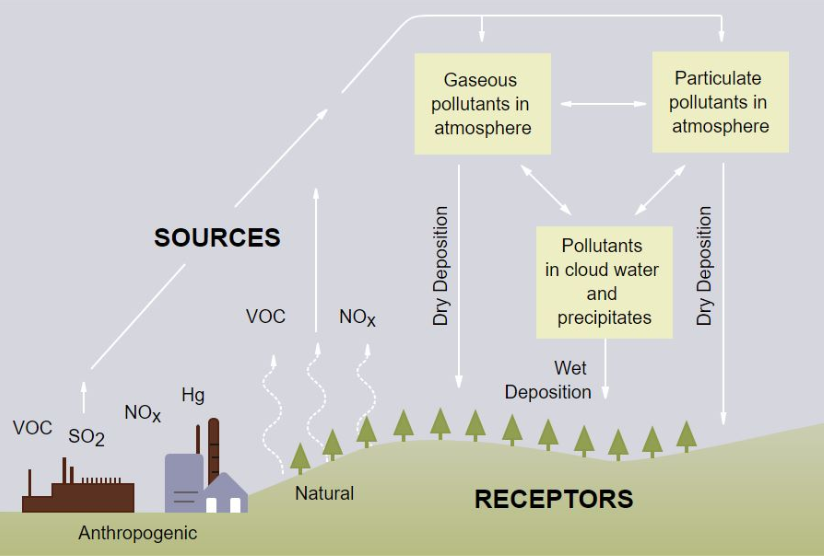
There is a wide variety of volatile organic compounds. While many of these chemicals occur in the atmosphere naturally, a significant portion of VOC emission come from manmade sources. Outdoor sources of VOCs can include the following2:
-
Traffic from cars, boats, and trains.
-
Chemical production and storage facilities.
-
The use of products that emit high VOCs concentrations.
Building materials are also known to release a number of VOCs. Paint, for example, is particularly hazardous and can contain a number of organic chemicals in high concentration for indoor spaces. Additionally, harmful VOCs, such as methylene chloride, can also be found in paint strippers3.
Acid rain
Acid rain is classified as any precipitation with acidic components. It can fall to the ground as wet as well as dry as rain, snow, fog, hail or acidic dust. Acid rain is primarily the result of nitrogen oxides (NOX) and sulfur dioxide (SO2) being emitted into the atmospheric environment and reacting with water. Acid rain can also be identified by its pH level, which is usually between 4.2 and 4.4, while normal rain has an approximate pH level of 5.6 (it is usually slightly acidic due to dissolved carbon dioxide). The primary sources of acid rain are (4):
-
Vehicles, such as cars.
-
Power stations that burn fossil fuels.
-
Oil refineries.
-
Manufacturing processes.
As mentioned above, acid rain can also fall to the ground dry as well as wet, in what is known as a ‘dry deposition’. This is where acidic particles fall from the atmosphere without any moisture. These particles, in the wrong circumstances, can form larger compounds that can be harmful to people’s health. When it finally does rain, these acidic compounds can be picked up and washed away to harm plant life and animals.
The most serious environmental damage acid rain can cause is to the planet’s ecosystem. Some of the most adverse effects take place in aquatic environments. At pH levels above 5, most fish eggs are unable to hatch and at lower levels, adult fish start to die with some lakes so acidic they don’t have any fish. Acid rain can also indirectly kill aquatic wildlife as well as it also can kill vegetation that the animals eat. Other animals that use the water can also be at risk and soil contamination is also possible.
Acid rain is also particularly harmful to trees in a number of ways. Firstly, when acid rain lands on soil, it can wash away vital nutrients the trees needs to survive. Secondly, it can release aluminum in the soil, which not only harms trees, but also other animals. Thirdly, in the form of acidic fog, leaves can be stripped away or damaged which makes it harder for them to absorb sunlight.
Additionally, acid rain can also have a significant impact on infrastructure people depend on, which can lead to some structures needing to be replaced or repaired. Sulfuric and nitric acid present in acid rain can damage the surfaces of buildings and statues, for example, as well as corrode metal, such as steel bridges (5).
Formation of ozone
While many people are aware that ozone helps protect our planet from harmful UV rays from the sun, at ground-level ozone is actually hazardous to our environment.
Normally, ozone or O3 is naturally formed high up in the atmosphere (stratosphere) when O2 molecules are separated into individual oxygen particles by UV radiation. These free oxygen particles then collide with other O2 molecules to become ozone (6). In the form of ozone, they absorb UV rays. Here is an example of how ozone is made:
O + O2 = O3
However, VOC emissions also contribute to the formation of ozone and particulate matter, but at ground-level, which can lead to smog. Aside from the adverse health implications to humans and animals, the effects of ozone on the environment can include increased chances of plants developing diseases, an inability to fight off pests and environmental stress, the reduced growth, and survival of tree seedlings, and reduced agricultural yields (7).
Ozone is created by VOCs and nitrogen oxides when they react with sunlight. Here is an example of how nitrogen dioxide can lead to the creation of ozone:
NO2 + Sunlight (UV rays) = NO + O
The free oxygen particle then attaches itself to an O2 molecule and becomes ozone.
On particularly hot and sunny days, such as those that occur during the summer months, outdoor air quality can decline significantly (8). When there is no wind, a phenomenon called “temperature inversion” can cause huge VOCs concentrations to happen in cities.
How to reduce the environmental effects of VOCs
By protecting plants that remove gaseous pollutants from the air, it is possible to reduce the effects these VOCs concentrations have on our health and environment.
Aside from being carcinogenic, cigarette smoke contains a number of VOCs. By reducing tobacco smoking or adopting alternative tobacco smoking methods that emit fewer chemicals into the air, it is possible to reduce the effects VOCs have on the environment.
Additional reading
People can also improve their indoor and outdoor air quality by developing an understanding of the following:
-
Knowing at what point exposure to VOCs becomes dangerous to people’s health is particularly important. This is usually determined by their threshold limit value. Bear in mind, different environmental agencies can have different standards.
-
People can refer to the IAQ standards and guidelines if they believe their health may be at risk from VOC emissions or other dangerous chemicals, such as carbon monoxide.
-
Finally, if people are educated on how to test for volatile organic compounds they can detect harmful VOC emissions and take action to improve protection from organic chemical in their homes or places of work.
Sources
1. http://www.businessdictionary.com/definition/volatile-organic-compound-VOC.html
2. https://www.tandfonline.com/doi/pdf/10.1080/08940630.1989.10466593
3. https://www.nicnas.gov.au/chemical-information/factsheets/chemical-name/methylene-chloride-in-paint-stripping
4. https://www.epa.gov/acidrain/what-acid-rain
5. https://www.epa.gov/acidrain/effects-acid-rain
6. http://www.theozonehole.com/ozonecreation.htm
7. https://www.canada.ca/en/environment-climate-change/services/managing-pollution/sources-industry/volatile-organic-compounds-consumer-commercial/overview.html
8. https://foobot.io/resources/heat-waves-make-air-quality-worse/#tab-con-2